Special Populations Underrepresented in Oncology Research: Challenges and Solutions to Engage the Hispanic Population
February 2018
Background - Formative Research
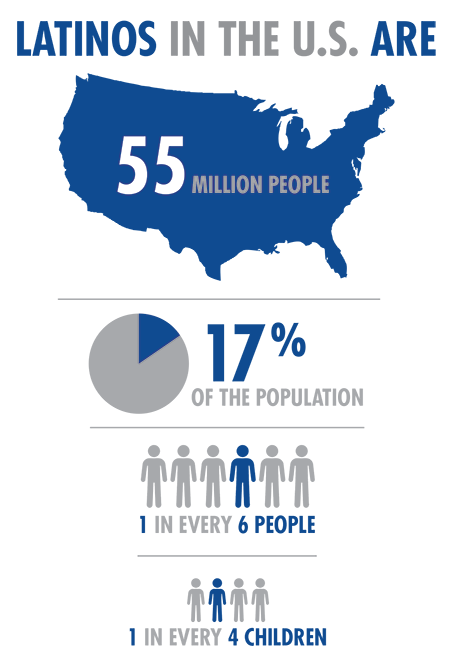
There are 55 million Hispanic persons living in the United States, making them the largest minority group in the country. Hispanics currently make up 17% of the US population1, representing a 43% increase over the past ten years2. This percentage is expected to grow to an estimated 30% of the population by 20503. A clear example of this trend is the State of Maryland, where, over the past three decades, the Hispanic population has more than tripled4. Notably, Maryland also houses the primary agency of the United States government responsible for biomedical and health-related research, The National Institutes of Health (NIH).
Despite the evidence that the Hispanic population is increasing at a formidable rate, they not only face disadvantages in health care access but are significantly underrepresented as participants in biomedical research. As a matter of fact, Hispanics make up 17% of the population but only 1% of clinical trial participants5, and fewer than two percent of National Cancer Institute-sponsored clinical trials focus on any racial/ethnic minority population6. Likewise, participation in HIV research studies is overwhelmingly from white males, although Hispanic and black men have higher rates of HIV infection.7.
TRI has always recognized the importance of enhancing ethnic diversity in oncology research, with particular attention paid to those identified as vulnerable and underserved. With this awareness, we provide a wide spectrum of services to the life sciences industry, such as the conception and implementation of participant recruitment, and retention campaigns.;
Importance of the Problem/ Why Advocate on the Issue?
In 2014, the leading cause of death for the Hispanic population was cancer (malignant neoplasms), followed by heart disease. In contrast, heart disease ranked first and cancer second for the non-Hispanic white and non-Hispanic black populations8. Overall, about one in three Hispanic men and one in three. Hispanic women will be diagnosed with cancer in their lifetimes. The lifetime probability of dying from cancer is one in five for Hispanic men and one in six for Hispanic women9.
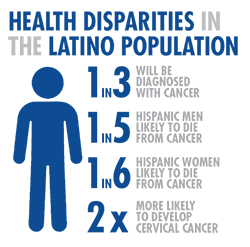
Some cancer types affect the Hispanic community more or less than others, or affect them differently. While Hispanics are less likely than non-Hispanic whites to be diagnosed with the most common cancers (lung, colorectal, breast, and prostate), they have a higher risk for cancers associated with infectious agents, such as liver, stomach, and cervix9. Notably, Hispanic women are almost twice as likely to have cervical cancer and 1.4% more likely to die from it as compared to non-Hispanic white women10.
Sadly, there is still a lack of understanding of cancer-specific biological factors among Hispanic people. The overwhelming majority of research on biological differences in cancer types has focused on African American women, as they are more likely to be diagnosed with aggressive tumors than non-Hispanic whites. However, relative to studies focusing on African American women, fewer studies have documented biological differences among Hispanic women’s breast cancer compared to other racial/ethnic groups11.
This is the case despite a 2007 population-based study from a cancer registry which reported that Hispanic breast cancer patients had higher BRCA1 mutation prevalence when compared to non-Ashkenazi Jewish whites, African Americans and Asian Americans, as well as suggesting a BRCA founder mutation specific to Mexican-American populations in the US12.
Cancer clinical trials, which permitted the majority of advancements in cancer treatment, may fail to address unique genetic and environmental factors of specific populations, such as Hispanics, that may place them at disadvantages for diagnosis and treatment in the future6. Unfortunately, diagnostic and therapeutic decisions determine that Hispanic patients' well-being and health outcomes are based on data gathered in populations of patients not representative of the Hispanic community who will bear the consequences of these decisions.
TRI has vast experience in conducting clinical trials that affect the Hispanic community. Our long-standing clinical trial expertise allows us to satisfy all clinical trials' regulations, protocol, and procedures.
Recruitment Challenges
Upon directives in legislation passed by Congress in the early 1990s, the NIH instituted policies aimed at increasing the representation of minority populations in clinical trials funded by the agency. The National Institute on Minority Health and Health Disparities is the lead organization at the NIH for planning, reviewing, coordinating, and evaluating minority health and health disparities research activities conducted by NIH Institutes and Centers. Low rates of minorities engaging in biomedical research is an issue that has been addressed through the NIH Revitalization Act of 1993. This has led to an increase in reporting of minority participation in NIH funded studies, from 1.5% in 1997 to 57% in 20116.
However, while the numbers on both reporting and minority participation have been on an upward trend since the implementation of the Act, the percentage of Hispanic participants in clinical research is still much lower than the representative proportion of the total U.S. population13. In fact, the rate of Hispanic participants enrolled in NIH-funded clinical studies in 2011 was only 8.2% (1,151,089) compared to 80% (10,765,968) of non-Hispanic participants14.
Many studies have tried to understand the reasons behind the underrepresentation of Hispanics in clinical studies and there appear to be multiple barriers to participation, such as stigma attached to clinical trials, lack of accessibility, inability to communicate in English, time constraints, need for transportation, need for child care during the visits, and the inability or fear of many patients regarding the loss of wages due to their participation in clinical trials11, 12. Lack of trust is also an issue with Hispanic patients who remember the oral contraceptive studies done with Hispanic women in the 1960s, during which patients neither received appropriate information about the study, nor were asked for informed consent.
There are also cultural taboos and myths among the Hispanic community regarding biomedical research. For example, some individuals may feel that by participating in clinical trials, they may be treated as human guinea pigs. Others may think that clinical trials are dangerous because doctors use new practices and medicines. Another common perception is that once they decide to participate in a clinical trial, they will not be able to change their minds.
>Recruitment Strategies
It is critical to recognize some contextual factors among the Hispanic community. For example, Hispanic people generally place a greater priority on faith than scientific data, and they like to include family as a part of their medical decisions. While this might seem like a barrier to physicians seeking individualized time with a patient, involving multiple family members is something clinical trial investigators must embrace if they want to recruit more Hispanic participants for oncology clinical trials, and get them to comply with treatment with investigational agents and follow-up visits.
Among the main interventions/activities that may be useful to increase Latino participation in oncology research: Generate and distribute educational material (in Spanish and English) about the importance of participation in clinical trials; create and record social media commercials (in English and Spanish) highlighting the importance of Latino participation in clinical trials; offer training sessions among health care providers on successful strategies to increase participation of their Latino patients in clinical trials.
Linguistically or culturally relevant materials facilitate awareness of the benefits of research participation in audiences that may not otherwise absorb generic messages. This point was demonstrated in a study where a video narrated in Spanish was used to recruit non-English speaking women to a research project on home safety visits. In this example, barriers of language and low literacy among Hispanic mothers were addressed through visual, language tailored, communication tools15.
The National Cancer Institute (NCI) has developed an Accrual Quality Improvement Program (AQuIP) Toolkit which provides guidance and helpful tips for creating high quality and professionally branded recruitment materials16. The AQuIP Toolkit assists clinical sites working with the NCI, Division of Cancer Prevention, to effectively recruit participants by providing trial teams with a detailed guide and turnkey resources. Among the preferred recruitment strategies are: conducting outreach to community representatives such as church leaders, asking for physician referrals in the Hispanic community, advertising on TV and in Hispanic print media, engaging the community on social networks, using the image of an individual of the same target minority group in the materials, and assigning someone of the same minority group as part of the recruitment team.
Based on our international work, TRI's project teams are especially knowledgeable about developing countries' regulations and cultural differences, and have developed effective remote (also called central) monitoring procedures and tools. To meet our clients' needs, TRI also provides Spanish translations as part of our clinical operations services.
;
Conclusion
Cancer is the leading cause of death among Hispanic people. Despite marked medical improvements in the treatment of cancer and increased survival rates for the general population17, members of this minority are more likely to be diagnosed with advanced stages of disease, have longer times to definitive diagnosis and treatment initiation, and experience poorer quality of life relative to non-Hispanic Whites18.
In spite of the rapidly increasing Hispanic population in the United States, the literature predominantly consists of studies targeting non-Hispanic Whites. As these trends indicate, there is an urgent need for more oncology research focusing on Hispanics in the U.S.
When clinical trial data are generated on diseases that particularly affect minority populations, the data are not necessarily applicable to all the key ethnic minorities who suffer a higher burden of these diseases if these minorities have been underrepresented or not included in the studies. In many instances, diagnostic and therapeutic decisions that affect patients' well-being and health outcomes are based on data gathered in populations of patients not representative of the patients who will bear the consequences of these decisions.
In spite of the previously discussed cultural barriers, research has shown the Hispanic population's strong willingness to participate in biomedical research19, 20. When motivation behind high willingness to participate was examined, two primary themes emerged. The first is benevolence - a desire to contribute to biomedical research for the purpose of helping others in their own community and beyond. Second, the opportunity to advance the prevention, diagnosis, and treatment of disease was highlighted as one of the key benefits of biomedical research19.
Despite the previously discussed socioeconomic and cultural factors that prevent Hispanic to participate in clinical trials, these barriers can be overcome, if the researchers are knowledgeable and display tact and cultural sensitivity.
In order to foment diversity, increase inclusiveness, and improve recruitment, TRI provides a diverse range of services, including marketing campaigns, graphics and multimedia, accrual monitoring and analytics. By supporting these strategies and encouraging involvement from underrepresented groups, TRI stands out as a pacesetter for diversity in oncology research. Tackling language and communication factors is clearly important for researchers striving to make the most impact with limited resources.
TRI acknowledges the importance of advocacy for diversity and inclusiveness in oncology clinical trials, because translation of research evidence into clinical practice is effective only in populations that are adequately represented.
Footnotes
- Colby SL, Ortman JM. (2014). "Projections of the Size and Composition of the U.S. Population: 2014 to 2060". Washington, DC: US Census Bureau.
- Ennis, S., M. Rios-Vargas and N. Albert. (2011). The Hispanic Population: 2010. U.S. Census Bureau, Population Division. Washington, DC: U.S. Government Printing Office.
- Passel J.S, Cohn D, (2008). "U.S. Population Projections: 2005-2050". Pew Research Center. Available at: http://www.pewhispanic.org/2008/02/11/us-population-projections-2005-2050/
- Hispanics in Maryland: health data and resources. (2013). Maryland Department of Health and Mental Hygiene. Office of Minority Health and Health Disparities. Available at: https://health.maryland.gov/mhhd/Documents/Maryland-Hispanic-Health-Disparity-Data.pdf
- The Society for Women's Health Research. "Dialogues on Diversifying Clinical Trials". 2011. Available at: http://swhr.org/portfolio/dialogues-on-diversifying-clinical-trials/
- Chen, M. S., P. N. Lara, J. H. T. Dang, D. A. Paterniti and K. Kelly (2014). "Twenty Years PostNIH Revitalization Act: Enhancing Minority Participation in Clinical Trials (EMPaCT): Laying the Groundwork for Improving Minority Clinical Trial Accrual." Cancer 120: 1091-1096.
- Shavers VL, Lynch CF, Burmeister LF. (2002). "Racial differences in factors that influence the willingness to participate in medical research studies". Ann Epidemiol. 2002 May; 12(4):248-56.
- Heron M. Deaths: Leading causes for 2010. National vital statistics reports; vol 62 no 6. Hyattsville, MD: National Center for Health Statistics. 2013.
- American Cancer Society. (2015) "Cancer Facts & Figures for Hispanics/Latinos 2015-2017". Atlanta: American Cancer Society. Available at: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/cancer-facts-and-figures-for-hispanics-and-latinos/cancer-facts-and-figures-for-hispanics-and-latinos-2015-2017.pdf
- CDC 2016. Cancer and Hispanic Americans. Health United States, 2015. Table 72. Available at: https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=61
- Yanes, B., Heather L., BuitragoD., Ramirez A., Penedo, Frank. (2016) "Cancer Outcomes in Hispanics/Latinos in the United States: An Integrative Review and Conceptual Model of Determinants of Health". J Lat Psychol. 2016 May ; 4(2): 114-129.
- Quinn, G., J. McIntyre and S. Vadaparampil (2011). "Challenges in recruiting Mexican women for cancer genetics research." Journal of Community Genetics 2(1): 43-47.
- Woodward AM, Dwinell AD, Arons BS. (1992). Barriers to mental health care for Hispanic Americans: a literature review and discussion. J Ment Health Adm. 1992 Fall; 19(3):224-36.
- The Endocrine Society. (2007). Increasing Minority Participation in Clinical Research. Available at: https://digital.lib.washington.edu/researchworks/bitstream/handle/1773/27576/Torres_washington_0250O_13800.pdf?sequence=1
- Hendrickson SG (2007) Video recruitment of non-English-speaking participants. West J Nurs Res; 29:232-242.
- Torres, S. (2014). Latinos and genetics: Addressing the disparity of Latino research participation in genetics. University of Washington. Available at: https://digital.lib.washington.edu/researchworks/bitstream/handle/1773/27576/Torres_washington_0250O_13800.pdf?sequence=1
- NIH Office of Research on Women's Health (ORWH). (2013). "Monitoring adherence to the NIH on the inclusion of women and minorities as subjects in clinical research. Comprehensive report: Tracking of clinical research as reported in fiscal year 2011 and fiscal year 2012." Retrieved July 28, 2014 from https://orwh.od.nih.gov/resources/pdf/Inclusion-ComprehensiveReport-FY-2011-2012.pdf
- The National Cancer Institute. Division of Cancer Prevention (2016). Accrual Quality Improvement Program (AQuIP) Toolkit. Available at: http://www.dcpaquip.com/Documents/MediaTempletes/DCP_AQuIPToolkit_Guidelines_Manual_v29Jan2016_v1_Web.pdf
- American Cancer Society. Cancer Facts & Figures 2015. 2015a. from https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2015/cancer-facts-and-figures-2015.pdf
- American Cancer Society. Cancer Facts & Figures for Hispanics/Latinos 2015-2017. 2015b. Retrieved July 14, 2015, from https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/cancer-facts-and-figures-for-hispanics-and-latinos/cancer-facts-and-figures-for-hispanics-and-latinos-2015-2017.pdf
- Ceballos RM, Knerr S, Scott MA, Hohl SD, Malen RC, Vilchis H, Thompson B. Latino beliefs about biomedical research participation: a qualitative study on the U.S.-Mexico border. J Empir Res Hum Res Ethics. 2014 Oct; 9(4):10-21
- Wendler D, Kington R, Madans J, Van Wye G, Christ-Schmidt H, Pratt LA, Brawley OW, Gross CP, Emanuel E. Are racial and ethnic minorities less willing to participate in health research? PLoS Medicine. 2006; 3(2):e19.10.1371/journal.pmed.-0030019
About the Author
Dr. Carlos M. Naranjo , is a bilingual, International Medical Doctor (MD) from Los Andes University in Venezuela. Currently, he is pursuing a Master's in Public Health at George Washington University. At TRI, he works as Pharmacovigilance Specialist, performing several tasks in support of clinical research including adverse event analysis and processing, serious adverse event reconciliation, preparation of IND safety reports for submission to the FDA, safety document or data analysis, and clinical trial site support. Please visit www.tech-res.com for more information about TRI's services.

